

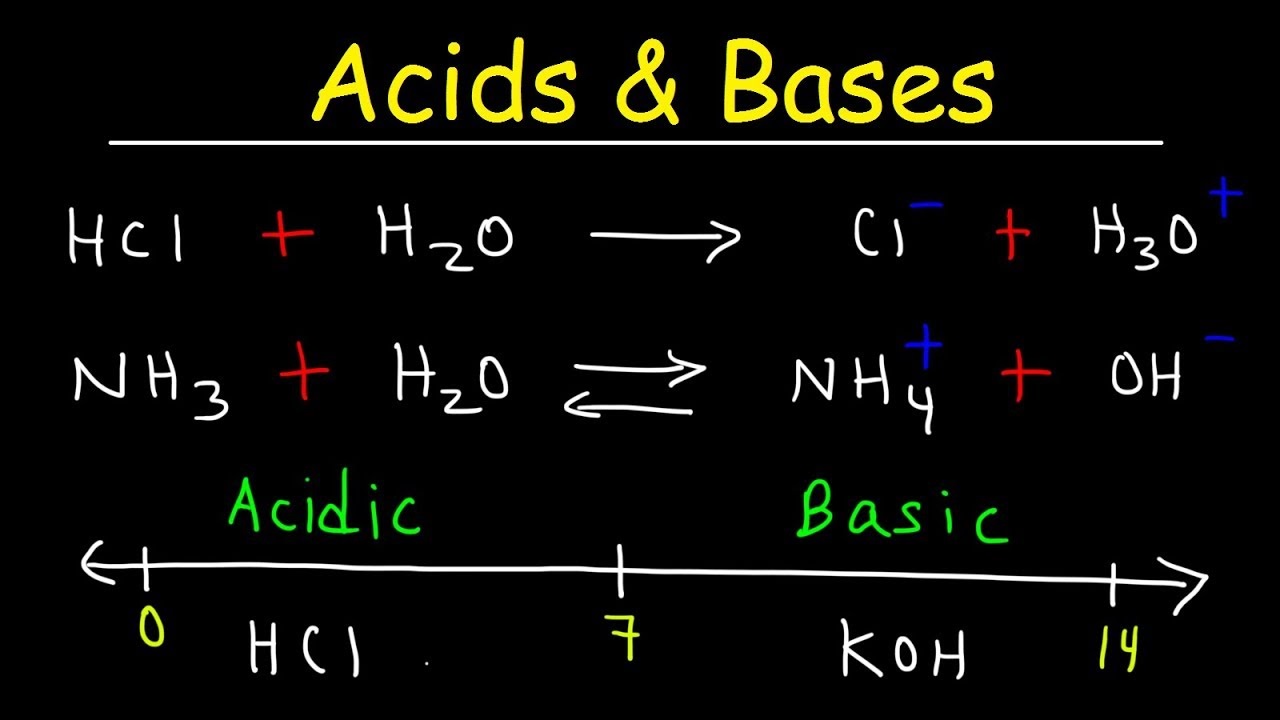
That is, all the acid molecules break up into ions and solvate (attach) to water molecules. Strong and Weak Acids/Bases Ī strong acid is an acid which dissociates completely in water. The conjugate pairs are distinguished with matching fonts. Again, acids are written in red, and the bases are written in blue. The conjugate acid is ammonium and the conjugate base is hydroxide. HA → H + + A − Īmmonia (basic) reacts with water (the acid). Arrhenius acid-base reactions can be summarized with three generic equations: It can be simply explained by these two points:īased on this definition, you can see that Arrhenius acids must be soluble in water.
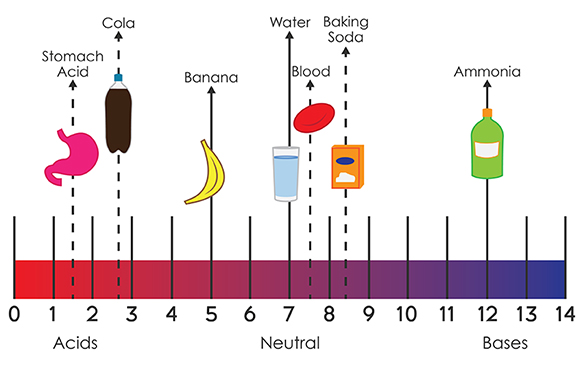
The Swedish chemist Svante Arrhenius published his theory of acids and bases in 1887. Over the years, much more accurate definitions of acids and bases have been created. He proposed that acids contained oxygen, although he did not know the dual composition of acids such as hydrochloric acid (HCl). The first scientific definition of an acid was proposed by the French chemist Antoine Lavoisier in the eighteenth century. Several different theories explain what composes an acid and a base. You may need to refresh your memory on naming acids. Chemicals that are acidic or basic are an important part of chemistry. Cleaning products like bleach and ammonia are bases. Some foods contain acid, like the citric acid in lemons and the lactic acid in dairy. Acid-Base Reaction Theories Īcids and bases are everywhere.


 0 kommentar(er)
0 kommentar(er)
